MARUM - Sediment Geochemistry - Leobener Str - D-28359 Bremen - Germany
Alkalinity (mol/l) = ( H+added - H+excess + H+ initial ) / v0
(1) Alkalinity (mol/l) = [(vHCl*cHCl) -10-pHfinal*(v0 +vHCl)/fH++ 10-pHstart * v0 /fH+] / v0
Examples
a)
pHstart = 8.02
vHCl = 1.32 ml (to pHfinal = 4.30)cHCl = 0.1 mol/l v0 = 50 ml fH+ = 0.8
Alkalinity = [(1.32/1000 * 0.1) – 10-4.3 * (50/1000 + 1.32/1000) / 0.8 + 10-8.02 * 50/1000 /0.8] / (50/1000)
Alkalinity = 0.00257 mol/l = 2.57 mmol/l
b)
pHstart = 4.95
vHCl = 22 ml ( pHfinal 3.912) cHCl = 0.001 mol/l v0 = 5 ml fH+ = 0.8
Alkalinity = [(22/1000 * 0.001) – 10-3.912 * (5/1000 + 22/1000) / 0.8+ 10-4.95 * 5/1000 /0.8] / (5/1000)
Alkalinity = 0.00358 mol/l = 3.58 mmol/l
If the initial pH is well above pH 5 the initial amount of H+-ions is neglectable and the equation simplifies to
Alkalinity (mol/l) = ( H+added - H+excess ) / v0
(2) Alkalinity (mol/l) = [(vHCl*cHCl) -10-pH*(v0+vHCl)/fH+] / v0
examples
pHstart = 8.02
vHCl = 1.32 ml (pHfinal = 4.30)cHCl = 0.1 mol/l v0 = 50 ml fH+ = 0.8
Alkalinity =[(1.32/1000 * 0.1) – 10-4.3 * (50/1000 + 1.32/1000) / 0.8 ] / (50/1000)
Alkalinity= 0.00257 mol/l = 2.57 mmol/l
b)
but :pHstart = 4.95
vHCl = 22 ml (pH final= 3.912)cHCl = 0.001 mol/l v0 = 5 ml fH+ = 0.8
Alkalinity = [(22/1000 * 0.001) – 10-3.912 * (5/1000 + 22/1000) / 0.8] / (5/1000)
Alkalinity = 0.00357 mol/l = 3.57 mmol/l
alkalinity calculation from different added volume / resulting pH value pairs of a real titration
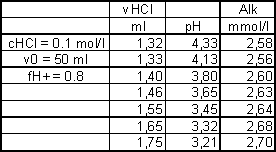
advantages of this method:
exact even for small initial sample volumes and when acid concentration is low (large titration volume to initial sample volume ratio), Fast since the final pH only needs to be well below 4.3 - no exact pH-value has to be reached. With some experience acid has to be added only once or twice.